Foreword
This is the second part of a series I hope to continue that deals with some interesting experiences I’ve had in my industrial hygiene journey. The first article covered the “Misnomer of Total Dust”. As with that post, I’d like to summarize something that once gave me fits early in my career. I lived, learned, and gained experience through my carbon monoxide mapping and monitoring work, and “having experience means you have made all the mistakes”. I hope that this article will save another hygienist a headache or two when it comes to gas detection.
Carbon monoxide is all around us. Contrary to the header image for this article, you can’t see or smell it (though sometimes it accompanies some odorous compounds or visible smoke). Typically every time something is burned (gasoline, wood, natural gas, garbage, etc.) there is going to be at least some carbon monoxide released into the environment. It is common enough that our homes are equipped with carbon monoxide detectors that alert us to unsafe concentrations.
A Quick Aside – Using Home Carbon Monoxide Detectors in Industrial Environments
I have seen many instances where a residential carbon monoxide detector is being utilized within an industrial environment. I would advise against this! These detectors are typically designed using Underwriters Laboratories Inc. Standard UL2034 alarm points:
400 ppm – Alarm between 4 and 15 minutes of exposure
150 ppm – Alarm between 10 and 50 minutes of exposure
70 ppm – Alarm between 60 and 240 minutes of exposure
The OSHA PEL for CO is 50 ppm. The ACGIH TLV for CO is 25 ppm. NIOSH has established an instantaneous 200 ppm ceiling level. It is possible you can be exceeding all of these limits while your box-store CO monitor sits silent! Even models with digital readouts often won’t display concentrations below 30 ppm, and calibration is nearly impossible.
Always use the best tool for the job. Residential carbon monoxide detectors belong in the home, not the factory.
Carbon Monoxide in All the Wrong Places
Carbon monoxide is also a common contaminant in the industrial world. Instead of being generated by a home furnace, dryer, oven, and automobile, the culprits are boilers, industrial heaters, propane forklifts, and open flame ovens.
There are many instruments available that are capable of measuring carbon monoxide. It is a sensor that is typically included in a standard 4-gas meter and is an indicator commonly referenced in indoor air quality studies. Several examples of the many available instruments are shown below:




These instruments are invaluable tools in evaluating carbon monoxide. The old methods required the use of detector tubes, which aren’t as versatile and could be affected adversely by environmental factors such as temperature. These data logging instruments are a welcome addition to a hygienist’s toolbox. But as with any instrument….they too have their flaws.
Unexpected Results during a Carbon Monoxide Survey
Let us look at a hypothetical survey activity at an industrial site. You are a health and safety manager for a large manufacturing company, and you know there are several sources of carbon monoxide that are currently controlled in your facility. The known sources are all either enclosed, exhausted, or both, in order to protect workers from carbon monoxide concentrations in the workplace. You decide to perform a full survey of the facility, to determine the effectiveness of your controls. You instruct a specialist on your team collect a number of readings throughout the facility floor with a carbon monoxide monitor.
Several hours later they return with results. The readings were near zero throughout (excellent!), except for one area of the facility that showed concentrations that exceeded the ACGIH TLV. This area was away from all the sources you were concerned about. The specialist even took a photo of the area with the elevated concentrations, shown below:

Your first reaction upon seeing the area with the elevated concentrations is that there must be a mistake. There are no combustion sources anywhere near your forklift battery charging area. That is in the shipping warehouse, far away from the production areas of the facility. The shipping forklifts were converted to battery (hence the picture) from propane long ago. This area is also far removed from the idling diesel trucks at the loading docks. After much thought, you decide to review the manual for the monitor to see if there is a possible explanation contained within.
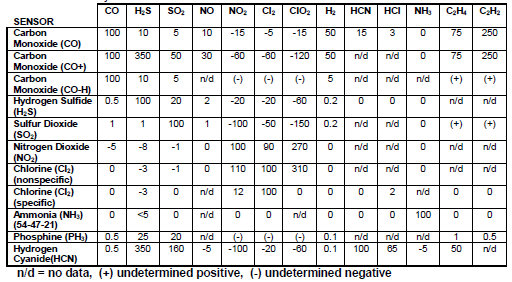
There is a table within the manual that shows chemicals that produce a positive or negative interference for the sensor. Eureka! The carbon monoxide monitor you have been using just so happens to have a significant positive interference for hydrogen gas (see the 50 under H2 for the carbon monoxide sensor, meaning 100 ppm of H2 would register as 50 ppm on your CO monitor). A quick search on OSHA’s website yields additional information: “When batteries are being recharged, they generate hydrogen gas that is explosive in certain concentrations in air”. Hydrogen gas doesn’t pose an exposure hazard (there is no PEL), but in high enough concentrations it poses a significant flammable hazard (the Hindenburg disaster provides notable evidence of that), at concentrations starting at ~4%. Thankfully, your battery charging area is well located, with high ceilings and nearby wall exhaust fans that prevent any buildup of hydrogen gas. Even with those controls, there was enough of the gas in the location to impact the carbon monoxide sensor.
Looking further into the table, you see a CO sensor marked (CO-H). That refers to a hydrogen null sensor, which reduces the cross-interference of hydrogen on the sensors readings (only a 5 ppm positive interference instead of 50 ppm). This may be an attractive option in future surveys, given the presence of hydrogen in your facility. It should also be noted that other chemicals also show as interfering agents for the monitor (particularly ethylene and acetylene, but also hydrogen sulfide, nitrogen oxides, and hydrogen cyanide).
There are other potential industrial processes that can produce hydrogen and therefore skew carbon monoxide readings. Many heat treating processes will use a hydrogen atmosphere within furnaces, often generated through the cracking of ammonia. Hydrogen can also used in the pouring of special castings, to manufacture magnesium, in fuel cells, to reduce iron ores and metal oxides, and to produce low temperatures (liquid hydrogen).
Whenever you have selected an instrument, become familiar with its weaknesses! Some instruments are highly sensitive to moisture (try using a flame ionization detector in the rain) while others will refuse to cooperate at low temperatures. Most instruments also have positive and negative interferences (as we saw earlier with carbon monoxide). Being prepared will save you significant confusion and embarrassment. These instruments don’t function like the Tricorder from Star Trek (see picture to the right), capable of instantly showing all sorts of amazing data at a seconds notice. [Though I do like to imagine that some thankless red-shirt Enterprise technician was tasked with calibrating all those devices before missions]

Conclusion
The takeaways from this text should be to:
- Use the proper tool for the job. Do not use residential carbon monoxide detectors in an industrial environment.
- Learn the instrument you are using. Most meters have interferences that can greatly skew readings both positively and negatively. Often they are designed for particular concentration ranges as well.
- Calibrate your instruments! Utilizing recently spanned/zeroed instruments (or better yet, having a canister of gas handy), can provide confidence that the meter is functioning properly.
The quality of the information obtained from an instrument is often impacted by the knowledge of the user. An improperly calibrated instrument will do more harm than good, and reacting to interferences can be a waste of resources. Do you know the reaction time for the sensor you are using? The concentration range? Be sure that you are confident in the data you are gathering!






